Πολύ ωραίο βίντεο, αν και η φωνη της ηταν εκνευριστικα παραμορφωμενη με αποτελεσμα να χτυπαει ασχημα στο αυτι, ενω δεν διευκρινησε το πως καταστρεφει ο οργανισμος τα υπολλειματα των mRNA και DNA στοιχειων και τι πιθανες παρενεργειες θα εχει απο αυτη τη διαδικασια.
Αυτο που μου αρεσε περισσοτερο ηταν η πληθωρα πηγων απο επιστημονικα περιοδικα και εγκυρες ιατρικες οργανωσεις (αν και σε μερικα με 30 citations θα εκραζε ο
@Snipereye 
)
Τσεκαρα τις πηγες, καθως ομως δεν ειχα χρονο να τις διαβασω ολες αναλυτικα, διαβασα αναλυτικα το abstract, το summary και τα conclusions. Τα conclusions σε ολες τις πηγες που αναφερει λενε οτι χρειαζεται χρονος κλινικων δοκιμων, κατι που προφανως δεν εγινε. Αυτο σε συνδυασμο με αυτο που ειπε ο
@Northlander για τη νομικη καλυψη των Φαρμακευτικων και η απλουστευμενη και αφελης ερμηνεια οτι οι Φαρμακευτικες δεν θα βγαλουν κατι στα γρηγορα γιατι θα τις κραξει ο κοσμος, με κανουν σαν ατομο αρκετα σκεπτικο πανω σε αυτην βεβιασμενη κινηση για μαζικο εμβολιασμο του πληθυσμου. Οπως και παλαιοτερα τα περισσοτερα κρατη ακολουθουν το σκεπτικο Οικονομια>Ανθρωποι... Μακαρι να κανω λαθος για την κινηση εμβολιασμου
15 Ιουλιου 2020
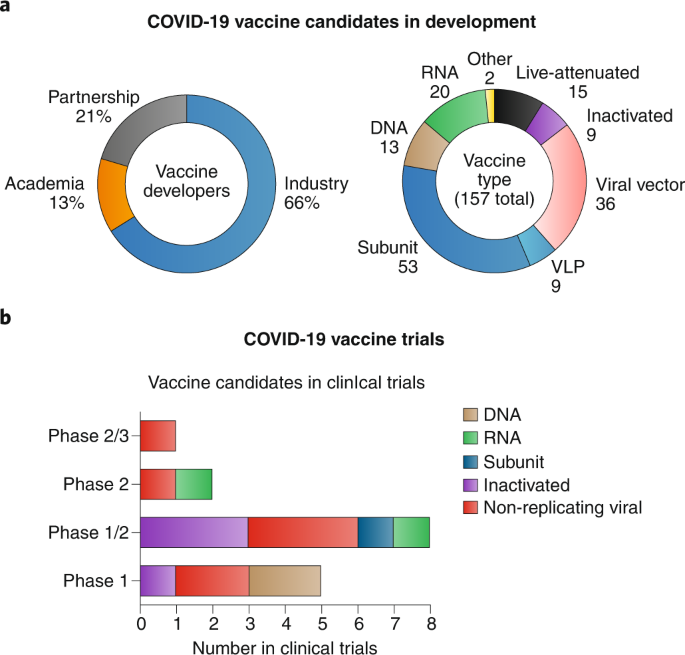
COVID-19 vaccine development and a potential nanomaterial path forward
This Review highlights current approaches to COVID-19 vaccine development, highlighting the role of nanotechnology, manufacturing and distribution.

www.nature.com
"While any vaccine is still months-to-years away from clinical reality, the parallel and rapid efforts from academic laboratories and industry provide hope for success. A plethora of nanotechnology platforms are being pivoted against SARS-CoV-2; while highly promising, many of these may be several years away from deployment and therefore may not have an impact on the SARS-CoV-2 pandemic."
3 Νοεμβριου 2020
Decoy nanoparticles protect against COVID-19 by concurrently adsorbing viruses and inflammatory cytokines
The COVID-19 pandemic caused by SARS-CoV-2 infection has led to more than 840,000 deaths worldwide as of August 31, 2020. Unfortunately, no licensed vaccine or specific treatment is available right now. Herein, we report a decoy nanoparticle against COVID-19. The decoy nanoparticles were...

www.pnas.org
"...However, further optimization and exploration are necessary. Scalability is always a key issue in the battle against such emerging pandemics. Mature gene editing, cellular vesicle purification, and fusion techniques promise rapid and large-scale production of nanodecoys. Another alluring feature of the nanodecoys is that the cellular vesicle components can be individually customized, providing a high degree of freedom in programmable development. Rare residual genetic materials in the nanodecoys may disturb host immunity, but short-term medication, together with evidence from our small-scale pilot safety study, could allay some of the safety concerns at this point in time. The host−virus affinity is also an important factor that needs to be accounted for. In the future, ACE2 variants with high affinity for viral S protein may further improve the blockade effects of nanodecoys on viral entry. Although detailed studies and further developments need to be carried out, our proof-of-concept work provides an approach to combat COVID-19 and other potential epidemics."
17 Ιανουαριου 2020
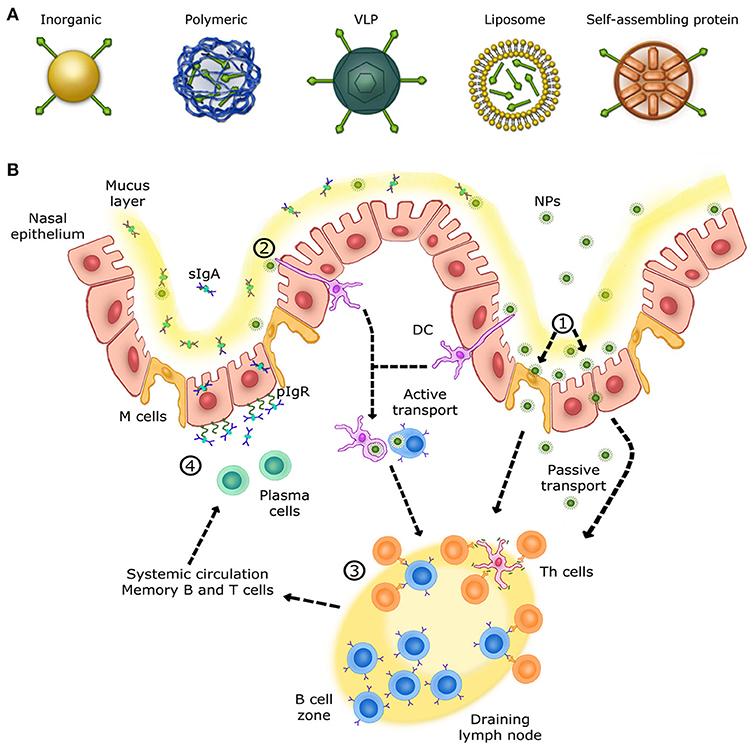
Nanoparticle-based vaccines
opportunities and limitations
"Some approaches inhibit the cytokine and chemokine cascade with DCs and T and B cells. NPs have great potential in immunology, although in-depth study of toxicity is necessary. In addition, NPs require molecules, such as antibodies or ligands, that bind to receptors on target cells to ensure that NPs only adhere to those cells. Any modification should be tested in cell lines or animals to prevent side effects or death."
7 Φεβρουαριου 2020
Nanotechnology in vaccine delivery
"With that being said, the development of any novel vaccine adjuvant or delivery platform, like any other pharmaceutical product, must prove its safety and tolerability before its approval.
Many challenges must be met before new classes of vaccines become available. Ideally, an adjuvant or delivery vehicle will have the ability to stimulate humoral, cellular and mucosal immune responses concurrently or discretely, depending upon the desired treatment strategy. The duration of the response should be long and the vaccine components should be easily metabolized by the body"
24 Ιανουαριου 2019
Nanoparticle-Based Vaccines Against Respiratory Viruses
The respiratory mucosa is the primary portal of entry for numerous viruses such as the respiratory syncytial virus, the influenza virus and the parainfluenza...

www.frontiersin.org
"Nonetheless, additional studies are still needed before their clinical translation. While intranasal vaccination of nanoparticles generates specific IgA antibody in the URT and leads to high survival rates in animal models, there are still limited studies on non-human primates, thus making nanoparticle's fate difficult to predict in a human URT. In addition, nanoparticle vaccines are generally functionalized with specific antigen(s), which result in an immune response targeted against these antigenic determinants"
12 Ιουνιου 2020
Vaccination strategies to combat novel corona virus SARS-CoV-2
"...preclinical studies of SAR-CoV-2 vaccine candidates may need to be performed in parallel with clinical trials. But in reality, SARS-CoV-2 vaccines will not be available for another
12–18 months because of the limitations such as unavailability of appropriate animal models
to check the efficacy and toxicity before going for the clinical trials.
Further it should comply with Good Manufacturing Practice (cGMP) to ensure the safety in humans."